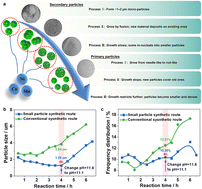
Comprehending the growth mechanism of precursors is crucial for the industrial fabrication of high-performance LiNi0.8Co0.1Mn0.1O2 (NCM811) cathode materials. Nonetheless, achieving precise control over particle size, morphology, and internal structure remains difficult due to limited understanding of precursor evolution during synthesis. This work tracks real-time reaction parameters and morphological evolution to investigate the growth behavior of Ni0.8Co0.1Mn0.1(OH)2 precursors. Variations in reactant concentration, feed rate, and consumption dynamics affect the observed three-stage growth mechanism of secondary particles. Approximately 2 μm-sized particles are initially generated through nucleation and subsequently aggregate into larger forms. As growth advances, the particle size distribution widens due to continuous nucleation and inhibited aggregation. Primary particles transition from nano-needle to rod-like forms, but their growth becomes increasingly restricted by limited energy and spatial constraints, leading to dense aggregation on pre-existing structures. The intermediate stage emerges as a crucial phase for controlling particle development. Fine-tuning during this stage effectively controls particle coarsening and promotes uniform secondary structures with intricate internal architectures. These observations provide valuable guidance for improving precursor synthesis, allowing for the scalable synthesis of Ni-rich cathode materials with enhanced performance.