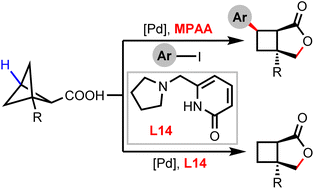
Bicyclo[3.2.0]heptane lactones represent an important scaffold in bioactive molecules. Herein, we report a diastereoselective synthetic disconnection to access bicyclo[3.2.0]heptane lactones from bicyclo[1.1.1]pentane carboxylic acids, which proceeds through palladium-catalyzed C–H activation and C–C cleavage processes. By using two different classes of ligands, MPAA and pyridone-amine, either all-syn arylated bicyclo[3.2.0]heptane lactones or non-arylated ones can be synthesized. The bicyclo[3.2.0]heptane lactone products were converted into multiple substituted cyclobutane, γ-lactone, and oxobicyclo[3.2.0]heptane derivatives to showcase the synthetic versatility of this method.
![Graphical abstract: Synthesis of bicyclo[3.2.0]heptane lactones via a ligand-enabled Pd-catalyzed C(sp3)–H activation cascade](http://pubs.rsc.org/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=D5SC00711A&imageInfo.ImageIdentifier.Year=2025)