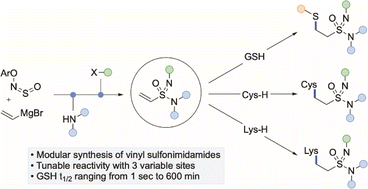
Covalent inhibitor design is dominated by the use of electrophilic acrylamide warheads. One limitation of acrylamides is that there are limited opportunities to modify their electrophilicity, and hence reactivity, by simple structural changes. Here we show that vinyl sulfonimidamides are effective electophilic groups for reaction with both sulfur- and nitrogen-based biologically relevant nucleophiles. The parent N–H vinyl sulfonimidamides are prepared in a single step from an aryl-ONSO reagent, a vinyl organometallic, and an appropriate amine. Imidic N-functionalisation is straightforward, providing a collection of electrophilic fragments of varied reactivity. We demonstrate that the electrophilicity of these new reagents can be modulated by choice of the imidic N-substituent, and when this is used in combination with alkene substituents, allows for a reactivity range both above and below that of the parent acrylamide.