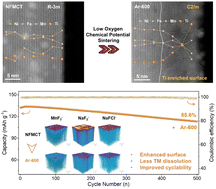
O3-Type layered oxides are promising Na-ion battery cathodes owing to their high theoretical capacity and facile synthesis. Increasing the upper cutoff voltage (V > 4.0 V vs. Na+/Na) is crucial for improving the practical capacity. However, irreversible electrolyte side reactions at high voltages impede their practical applications. In this work, we develop a low oxygen chemical potential (LOCP) sintering strategy to modify the O3-type cathode, creating Ti-rich surfaces through structural reorganization. This surface engineering significantly enhances the interfacial stability between the cathode and the electrolyte, and the optimized cathode delivers an initial discharge capacity of 146.7 mA h g−1 with 85.6% capacity retention after 500 cycles in a pouch full-cell. Mechanistic studies reveal that LOCP sintering induces surface oxygen vacancies while enabling Na deintercalation and surface sodium residual accumulation. These oxygen vacancies promote bulk-to-surface Ti migration alongside Mn valence reduction, ultimately driving structural phase transitions. DFT calculations confirm that oxygen vacancies reduce Ti migration barriers, elucidating the atomic-scale transformation mechanism. This work sheds light on surface engineering of O3-type layered oxides and provides valuable insights for developing high-voltage sodium-ion batteries with extended cyclability.