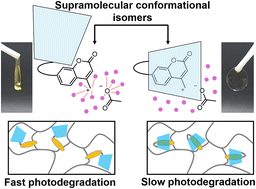
Photolabile polymer materials have attracted considerable scientific and societal attention owing to the spatiotemporal control of light. However, easily photoreactive polymers are intrinsically unstable, leading to a tradeoff between their reactivity and stability. This article reports a proof-of-concept for reversible switching between photostable and photolabile states in polymer network materials enabled by the supramolecular transformation of the kinetically stable pseudo[1]rotaxane. A photocleavable coumarinylmethyl ester derivative, covalently linked with permethylated α-cyclodextrins, can be switched between insulated and uninsulated structures through conformational isomerization by altering the solvent polarity and heating. The hydrophobic environment of the cyclodextrin in the insulated structure partially inhibits polar solvation of the contact ion pair, an intermediate in the photocleavage reaction, thereby decreasing the photoreactivity of the coumarinylmethyl ester. Consequently, polymer network materials crosslinked with the insulated structure exhibit photostability, whereas the corresponding uninsulated structure is photolabile, demonstrating the reversible control of material photoreactivity via supramolecular conformational transformations.
![Graphical abstract: Supramolecular conformational control of photolability in polymer networks crosslinked with a kinetically stable pseudo[1]rotaxane based on a coumarinylmethyl ester](http://pubs.rsc.org/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=D5SC01641J&imageInfo.ImageIdentifier.Year=2025)