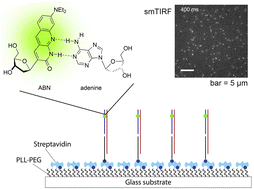
Fluorescent nucleobase analogues (FBAs) have emerged as powerful tools for understanding nucleic acid systems at the molecular level. However, their application at the single-molecule level has been limited by low brightness and an incomplete understanding of how local chemical environments affect their properties. In this study, we investigate the bright fluorescent pyrimidine analogue ABN in duplex DNA oligonucleotides and study its single-molecule applications. Time-resolved fluorescence spectroscopy reveals its unique tautomeric behavior, including photo-induced double proton transfer, influenced by base-pairing partners. This tautomerization directly impacts ABN’s quantum yield and spectral characteristics. By favoring a high quantum yield thymine-like tautomer through base pairing, surface-immobilized ABN-containing DNA duplexes are readily observed as bright spots using single-molecule fluorescence microscopy, exhibiting well-defined single-exponential bleaching kinetics. The brightness and photostability are enhanced by oxygen depletion. These results demonstrate that ABN is unique among FBAs in enabling single-molecule fluorescence studies of oligonucleotides using a standard microscopy setup.