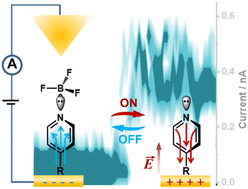
The non-faradaic application of electric fields generated at the surface of charged electrodes to polarize bound molecules, also termed as electro-inductive effects, have recently attracted increasing attention in modifying the chemical reactivity of molecules in electrosynthesis. Herein, we applied this electro-inductive effect to control the Lewis adduct formation and dissociation between BF3 and pyridine N of heterocycles to realize single-molecule contact switching. In situ single-molecule conductance measurements, in situ Raman analysis and theoretical calculations clearly show that the outward electric field along the positively-charged electrode surface polarizes adsorbed molecules to withdraw electron density from the terminal pyridine N, which weakens the N–BF3 Lewis bond for dissociation upon applied positive potentials. The released unbounded pyridine N can connect the molecule into a molecular circuit for electron transfer (considered as the “ON” state). Meanwhile, the inward electric field along the negatively charged electrode surface promotes the formation of an N–BF3 Lewis bond, leading to breaking of the molecular circuit (considered as the “OFF” state). Combined with the optimization of BF3 concentration from the equilibrium BF4− ⇌ BF3 + F−, the electro-inductive effect can reversibly switch single-molecule conductance in conductance measurements and tunnelling currents in I–V measurements.