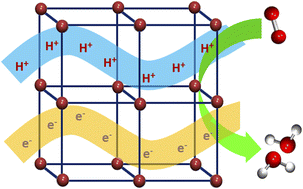
Proton coupled electron transfer (PCET) is considered as the elementary step of several chemical, electrochemical and biological processes and thus the development of dual conducting materials has recently become a major focus in Chemical Science. Herein, we report the highly selective electrocatalytic oxygen reduction to water by the stable dual conducting metal–organic material (MOM) [Cu(INA)2(H2O)4] (INA = isonicotinate). Structural analysis reveals the important role of both, hydrogen bonding and π-interactions, in the formation of a supramolecular 3D network. Theoretical calculations show that hydrogen bonding interactions among the coordinated water molecules and deprotonated carboxylate oxygen atoms induce proton transport (2.26 ± 0.10 × 10−5 S cm−1 at 98% RH) while weak intermolecular π-interactions (π–π and anion–π) provide the pathway for electron transport (1.4 ± 0.1 × 10−7 S cm−1 at 400 K). Such dual proton and electron conductivity leads to a selective oxygen reduction reaction (ORR) to water in an alkaline medium. To the best of our knowledge, this is the first report on electrocatalytic ORR by a dual-conducting metal–organic material.