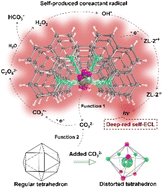
Self-electrochemiluminescence (self-ECL) can deal with the problems of limited electron transport efficiency, reduced signal stability, and redundant ECL processes. A feasible solution is to assemble the luminescent components and coreactant radicals together. Here a self-ECL material was designed relying on a europium-based metal–organic framework (Eu-MOF, ZL-2) with 1,10-phenanthroline and CO32− as ligands under strong alkaline conditions. Upon oxidation of the CO32− ligand to produce C2O62−, OH˙ could be generated to act as a coreactant radical, which reacted with the luminophore radical of oxidized ZL-2 to realize self-ECL without an extra coreactant. The coordination of the CO32− ligand induced Eu3+ to occupy some sites in the tetrakaidecahedron structure of ZL-2, which caused the structure to distort, and thus triggered the unusual electric-dipole transfer transition 5D0–7F4. Therefore, ZL-2 was endowed with deep-red luminescence in the first near-infrared (NIR-I) region, which filled a gap in self-NIR-ECL applications. This discovery suggested a novel promising dual-function mechanism of the CO32− ligand, undoubtedly broadening the application and development of MOFs in the ECL field.