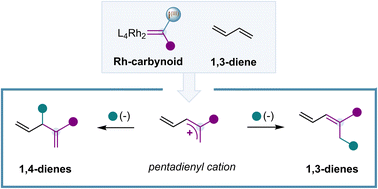
Herein, we report the first catalytic single-carbon insertion to 1,3-dienes with Rh(II)-carbynoids. The skeletal editing process is based on the catalytic generation of a Rh-carbynoid that promotes the insertion of a cationic monovalent carbon unit (:+C–R) into the C(sp2)–C(sp2) bond of the 1,3-diene, leading to a pentadienyl cation. Regioselective attack on the latter species leads to the formation of multi-substituted 1,3-dienes or 1,4-dienes with a broad range of carbon and heteroatomic nucleophiles.