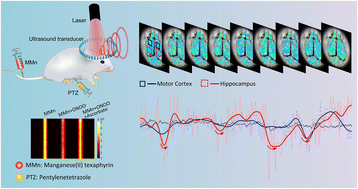
Real-time visualization and tracking of epileptic seizures are important for studying epilepsy pathogenesis and treating epilepsy; however, the requisite sensing is extremely challenging, primarily due to the transient and intricate nature of neural activity associated with epilepsy. The onset of epilepsy is closely correlated with increases in peroxynitrite (ONOO−) levels, a reactive nitrogen species that can serve as a biomarker for epilepsy. However, the fleeting biological half-life and high reactivity of ONOO− has historically impeded its direct visualization within the epileptic brain. This study explores the efficacy of manganese(II) texaphyrin (MMn), a water-soluble and stable expanded porphyrin, in dynamically sensing ONOO− and providing real-time tracking of epileptic seizures using a custom-built photoacoustic imaging (PAI) setup. UV-vis spectral analyses established the preferential sensitivity of MMn to ONOO− over other reactive oxygen species (ROS), as well as its effectiveness through multiple usage cycles when rejuvenated via reaction with suitable reducing agents. This selectivity was recapitulated in vitro as determined through PAI experiments. In vivo application of this technique revealed that MMn administered intravenously crosses the blood–brain barrier (BBB) in a pentylenetetrazole (PTZ)-induced epilepsy mouse model and provides an observable 14.1 ± 3.7% reduction in photoacoustic (PA) signal intensity within the hippocampal region during epileptic seizures. Multiple decreasing–increasing cycles of PA signal intensity could be detected in the hippocampal region in this model; the observed effect thus mirrors closely the course of epileptic seizures inferred from mouse tail curling. Similar cyclical patterns were also seen in the motor cortex, a finding consistent with the extensive spread of epileptic activity throughout the brain. To the best of our knowledge, the present investigation represents the first real-time visualization and tracking of epileptic seizures using a peroxynitrite-specific sensing probe in combination with photoacoustic imaging (PAI). This approach enables deeper brain imaging while simultaneously capturing dynamic ONOO− fluctuations, offering biochemical insights into epilepsy pathogenesis. By integrating deep-tissue imaging with neurochemical monitoring, this method lays the foundation for potential advances in epilepsy management and treatment.