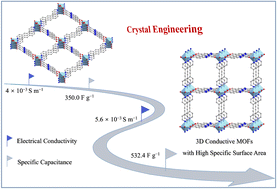
3D conductive metal–organic frameworks (c-MOFs) have emerged as a promising candidate for advancing energy storage due to excellent inherent electrical conductivity, efficient ion diffusion through open channels and high-density active sites. However, the facile preparation of 3D c-MOFs remains a great challenge. In this study, we developed two new 3D nitrogen-rich Ni-based c-MOFs (Ni-BPE and Ni-BPA) via the reaction of Ni2+ ions, 2-(3,5-dicarboxyphenyl)-6-carboxybenzimidazole (H3L) and 1,2-di(4-pyridyl) ethylene dipyridyl (BPE) or 1,2-di(pyridin-4-yl) ethyne (BPA). The nitrogen-rich ligands, featuring pyridyl and benzimidazole units, extend the π-conjugation system, contributing to the high conductivity of Ni-BPE. Furthermore, compared to flexible BPE with a carbon–carbon double bond, the rigid BPA with a carbon–carbon triple bond can endow MOFs with a stronger π-electron delocalization. Owing to the advantageous properties enabled by crystal engineering, Ni-BPA exhibited an excellent electrical conductivity (σ = 5.64 × 10−3 S m−1), which is 40% higher than that of Ni-BPE, accelerating electrochemical redox kinetics. Theoretical calculations confirmed the effect of electronic structure modulation on conductivity. Correspondingly, Ni-BPA produced a high specific capacitance of 532.4 F g−1 (266.2 C g−1) at 1 A g−1, surpassing Ni-BPE by 52.1%. Notably, the Ni-BPA//AC device maintained excellent cycle stability with a capacitance retention of 91.9% and a high coulombic efficiency of 98.6% after 10 000 cycles.