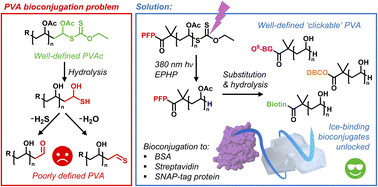
The (bio)conjugation of polymers onto proteins enhances their pharmacokinetics and stability, most commonly using PEG (polyethylene glycol), but there is a need for alternatives. Poly(vinyl alcohol), PVA, is a water-soluble, biocompatible and environmentally degradable polymer, which also has the unique function of ice recrystallisation inhibition (IRI) which can aid the cryopreservation of biologics. Site-specific PVA bioconjugation (“PVAylation”) is underexplored due to the challenge of obtaining homogenous mono end-functional PVA. Here we show that following deprotection of the acetate (from the precursor poly(vinyl acetate)), the concurrent xanthate end-group reduction leads to a diversity of ambiguous end-groups which prevented precision conjugation. This is overcome by using a photo-catalyzed reduction of the omega-terminal xanthates to C–H, which is orthogonal to active-ester bioconjugation functionality at the alpha-chain terminus, demonstrated by MALDI-TOF mass spectrometry. This strategy enabled the preparation of well-defined mono-functional PVA displaying alkyne, biotin and O6-benzylguanine chain-end functionalities, which are each then used for covalent or non-covalent site-specific modification of three model proteins, introduce ice-binding function. These results will enable the synthesis of new bioconjugates containing PVA and be of particular benefit for low-temperature applications.