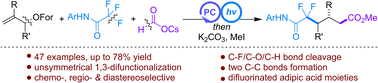
A transition-metal-free protocol for the unsymmetrical radical 1,3-difunctionalization of alkenes has been established for the first time in the form of 1,3-difluoroalkylcarboxylation by a photocatalytic radical three-component reaction of allyl formates, trifluoroacetanilides, and cesium formate. This reaction employs formate as the carboxylating reagent and trifluoroacetanilide as the difluoroalkylating reagent via C–F bond activation. As a result, a series of previously inaccessible unsymmetrical difluorinated adipic acid derivatives can be easily and efficient prepared. Mechanism studies reveal that triple kinetic-controlled radical self-ordering is the key to this unique reaction. This radical sorting involves the fast initiation of a CO2 radical anion and its chemoselective addition and reduction, followed by the slow generation of a fluoroalkyl radical and its chemo-/regioselective addition. Notably, this strategy is also suitable for the 1,3-difluoroalkylcarboxylation of unsymmetrical and cyclic alkenes through diastereoselectively constructing two or three consecutive stereocenters.