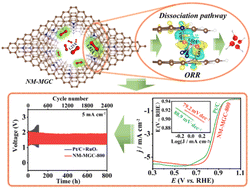
O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond activation determines activities of oxygen reduction reaction (ORR) catalysts, and developing metal-free catalysts that outperform precious metals remains challenging. Herein, we demonstrate the mechanochemical polymerization of pyrrole tandem carbonization for designing nitrogen-containing, micropore-penetrated multilayer graphene crystals (NM-MGCs) with abundant barrier-free nanochannels for O2, leading to high exposure of carbon atoms adjacent to graphitic nitrogen. Due to the ordered multilayer structure with steady layer-by-layer van der Waals interaction, the resultant exposed interlayer carbon atoms exhibit much improved ability to activate O2 through adsorption-configuration-induced O
O bond activation determines activities of oxygen reduction reaction (ORR) catalysts, and developing metal-free catalysts that outperform precious metals remains challenging. Herein, we demonstrate the mechanochemical polymerization of pyrrole tandem carbonization for designing nitrogen-containing, micropore-penetrated multilayer graphene crystals (NM-MGCs) with abundant barrier-free nanochannels for O2, leading to high exposure of carbon atoms adjacent to graphitic nitrogen. Due to the ordered multilayer structure with steady layer-by-layer van der Waals interaction, the resultant exposed interlayer carbon atoms exhibit much improved ability to activate O2 through adsorption-configuration-induced O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond dissociation in the ORR with approximately zero energy barrier. Thus, the NM-MGCs show record-breaking ORR performance among metal-free catalysts, which can be fabricated as air cathodes for both flow and flexible Zn–air batteries, exhibiting high maximum power density, high specific capacity (815.30 mA h gZn−1 at 5 mA cm−2), and extraordinary long-term cycling durability (>800 h) and round-trip energy efficiency (63.7%). The overall performance of Zn–air batteries assembled using the NM-MGCs surpass those of commercial Pt/C (20 wt%) and many literature-reported metal and/or metal-free electrocatalysts.
O bond dissociation in the ORR with approximately zero energy barrier. Thus, the NM-MGCs show record-breaking ORR performance among metal-free catalysts, which can be fabricated as air cathodes for both flow and flexible Zn–air batteries, exhibiting high maximum power density, high specific capacity (815.30 mA h gZn−1 at 5 mA cm−2), and extraordinary long-term cycling durability (>800 h) and round-trip energy efficiency (63.7%). The overall performance of Zn–air batteries assembled using the NM-MGCs surpass those of commercial Pt/C (20 wt%) and many literature-reported metal and/or metal-free electrocatalysts.
![Graphical abstract: Multilayer graphene crystals with enhanced performances in oxygen reduction and zinc–air batteries via interlayer carbon promoted O [[double bond, length as m-dash]] O bond dissociation](http://pubs.rsc.org/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=D5SC02570B&imageInfo.ImageIdentifier.Year=2025)