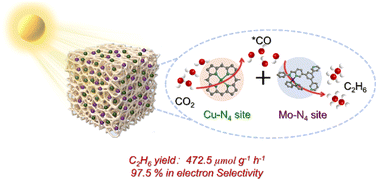
Photocatalytic CO2 reduction for the production of multicarbon products has emerged as a green and sustainable technology, which shows great potential and cost-effectiveness. However, photocatalytic synthesis of two-carbon (C2) compounds is quite challenging due to the high activation barrier of the C–C coupling reaction and low content of intermediates. Herein, inspired by the tandem synthesis in multi-enzyme reactions, Cu–N4 and Mo–N4 active sites have been designed and integrated in CuPor-POP-Mo as cascade dual metal sites for efficient photocatalytic reduction of CO2 to ethane (C2H6) for the first time. Significantly, an excellent C2H6 production rate of 472.5 μmol g−1 h−1 and a high product selectivity of 87.5% (electron selectivity ∼97.5%) have been achieved, which are the record high values in photocatalytic C2H6 production by using porous polymer catalysts. In situ spectral characterization studies and DFT calculations indicate that the Cu site enhanced the localized surface coverage of *CO on CuPor-POP-Mo, while the Mo site of the photocatalyst triggered the C–C coupling of *CO intermediates, and the energy barrier of which was synergistically lowered by Cu and Mo sites, resulting in highly effective C2H6 production. This work develops novel metal sites for ethane production and enables precise modulation of *CO intermediate coverage to decrease the energy barrier for *OCCO generation, thus opening a new pathway for highly selective photocatalytic CO2 reduction toward value-added chemicals and fuels.