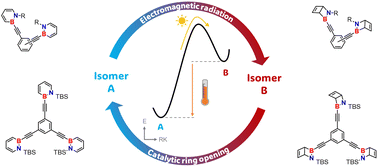
The reversible photoisomerization of 1,2-dihydro-1,2-azaborinines (BN benzenes) to their Dewar isomers (2-aza-3-borabicyclo[2.2.0]hex-5-enes) provides a promising platform for molecular solar thermal (MOST) energy conversion, storage, and release. We examine how energy density can be optimized by bundling multiple dihydroazaborinine units into a single molecule and explore how properties change depending on the connectivity of these units. Remarkably high molar energy densities of up to 644 kJ mol−1 were obtained, as well as a significant decrease in the half-life of the storage state in the order of ortho > meta > para. Moreover, the absorption is shifted from the UV-C of the parent 1,2-dihydro-1,2-azaborinine into the UV-A region. The investigated dyads and triades meet several criteria for an ideal molecular solar thermal storage material.