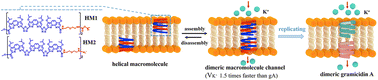
Structural simulation of natural ion channels remains a challenging topic. To fabricate artificial ion channels structurally resembling natural gramicidin A (gA), we prepared a type of precise hollow helical molecular channel by means of a modular synthesis strategy. Helical molecules are able to form 2.9 nm membrane-spanning channels through dimeric π-stacking assembly and efficiently accelerate ion transmembrane transport, with ultrahigh transport activity of up to 28 nM. Among these molecular channels with transmembrane structures similar to gA, one of them significantly exceeds natural gA for potassium ion transport, while another one exhibits the same proton transport activity as natural gA under identical conditions. Moreover, we found that the positive charges near the entrance of the channel reduce the potassium transport rate of the channel but significantly promote proton transport. In addition, a molecular channel with terminal amine groups shows pH-regulated ion transport function. This is the first example of structural replication of natural gA, in which helically folded molecules with assembled dimeric structure yield fantastic ion transport properties.