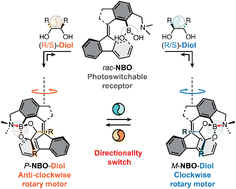
Chiral sterically overcrowded alkenes are potential candidates for artificial light-driven rotary molecular motors (LRMMs), which perform a full 360° unidirectional rotation around the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond through a series of photochemical and thermal isomerization processes. However, the majority of the reported LRMMs adopt an intrinsic chirality (i.e., an integration of the chirality center with the photoresponsive unit), which hampers the effective gating of their rotary direction through chirality switching. Herein, we report a new sterically overcrowded alkene equipped with a boronic acid receptor for dynamic covalent bonding with chiral vicinal diols, enabling it to function as an extrinsic chirality-based LRMM. The dynamic boronic acid-chiral diol B–O bonding not only implements the extrinsic chirality to induce a helical preference in the alkene backbone but also facilitates chirality switching through diol exchange to reverse the rotation direction. This work demonstrates that dynamic covalent bonding for extrinsic chirality implementation is an effective strategy for designing direction-switchable LRMMs, paving the way for more sophisticated molecular motors with applications in complex (bio)environments.
C bond through a series of photochemical and thermal isomerization processes. However, the majority of the reported LRMMs adopt an intrinsic chirality (i.e., an integration of the chirality center with the photoresponsive unit), which hampers the effective gating of their rotary direction through chirality switching. Herein, we report a new sterically overcrowded alkene equipped with a boronic acid receptor for dynamic covalent bonding with chiral vicinal diols, enabling it to function as an extrinsic chirality-based LRMM. The dynamic boronic acid-chiral diol B–O bonding not only implements the extrinsic chirality to induce a helical preference in the alkene backbone but also facilitates chirality switching through diol exchange to reverse the rotation direction. This work demonstrates that dynamic covalent bonding for extrinsic chirality implementation is an effective strategy for designing direction-switchable LRMMs, paving the way for more sophisticated molecular motors with applications in complex (bio)environments.