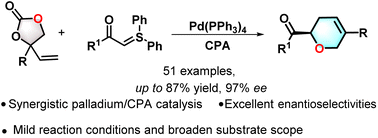
An effective method for the synthesis of dihydropyrans through synergistic palladium and chiral phosphonic acid catalysis was reported. This protocol proceeded under mild reactions and provided dihydropyrans in up to 87% yield and up to 97% ee. Meanwhile, various derivations such as oxidation, Wittig-reaction, reductions, nucleophilic substitution, and Baeyer–Villiger were accomplished to furnish interesting compounds. To gain insight into the reaction mechanism, nonlinear relationship experiments and Hammett plot experiments were carried out. In addition, a range of products (3i, 4b, 4f, 4g, and 4j) accessible from this method exhibit various anti-inflammatory activities on NO and ROS inhibition.
![Graphical abstract: Enantioselective [5 + 1] cycloaddition of sulfur ylides and vinylethylene carbonates via synergistic palladium/chiral phosphonic acid catalysis](http://pubs.rsc.org/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=D5SC01050K&imageInfo.ImageIdentifier.Year=2025)