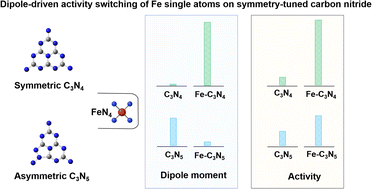
Efforts in designing efficient polymer-based single atom photocatalysts (SAPs) have primarily focused on selecting specific metal atoms with tailored geometries and properties to control functionality. However, the impact of the light-harvesting units that bridge these single metal atoms, crucial for light absorption and energy transfer, has been largely overlooked. In this work, two carbon nitride (CN)-based iron SAPs with a similar FeN4 coordination environment are synthesized: triazine-based CN (C3N4) and nitrogen-rich triazole-based CN (C3N5), differing in the unit cell structure. C3N5 exhibits better photocatalytic ozonation performance than C3N4 due to its unit cell asymmetry, which induces a dipole field that facilitates charge transfer. The addition of iron single atoms breaks the symmetry of C3N4 to enhance the dipole moment, while they weaken the separation and migration of bulk charge carriers in the Fe-C3N5 SAP. The iron atoms act as active sites in both Fe-C3N4 and Fe-C3N5 SAPs, accelerating interfacial reaction kinetics. These findings demonstrate the importance of the light-harvesting unit structures of CN-based SAPs in regulating photogenerated charge kinetics and offering valuable insights for the rational design of effective photocatalysts.