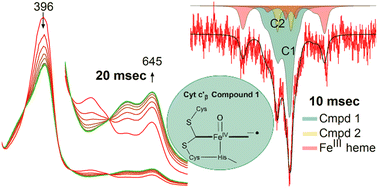
Compound 1 is a key intermediate in a large group of enzymes containing heme b including cytochromes (cyt) P450, peroxidases and catalases. The extreme reactivity of this highly oxidized species has made its trapping and characterization challenging. Cyt c′β of the ammonia-oxidizing bacterium Nitrosomonas europaea is an enzyme containing heme c and a member of the P460 superfamily of cytochromes. Herein, a compound 1 species within the P460 superfamily was trapped and spectroscopically characterized with stopped-flow absorption, EPR and variable-field Mössbauer spectroscopy. Like horseradish peroxidase (HRP), Cyt c′β has an axial His coordinated to the heme iron with the compound 1 species exhibiting a green color with an absorption spectrum similar to that of HRP. Compound 1 from Cyt c′β features an antiferromagnetic exchange coupling between an S = 1 iron(IV)-oxo center and an S = ½ porphyrin radical, but with an exchange coupling constant that is significantly greater than that for HRP. The large variation in the exchange constant for both enzymes, despite both having the same axial ligand, indicates that this value is not strongly correlated to the axial ligand identity. Although the reaction of Cyt c′β with H2O2 was found to be significantly slower than other peroxidases, the lifetime of the compound 1 species was comparable to other heme enzymes that oxidize a variety of substrates, suggesting that the in vivo function of cyt c′β may not be relegated solely to the clearance of H2O2. Given its presence in an ammonia-oxidizing bacterium, cyt c′β could oxidize nitrogen containing substrates.