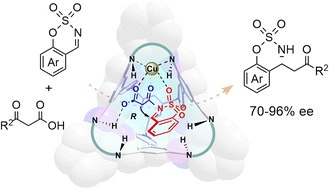
Using supramolecular chiral cages to create a favorable chiral environment can effectively address the limitations of traditional metal asymmetric catalysis in controlling chiral catalytically active centers. However, achieving harmonious interactions among the molecular cage, the metal, and the substrate within the cavity remains a significant challenge. To overcome this, we have designed a pyridinium-modified, chiral-diamine-functionalized cage with a distinct bowl-shaped geometry. This structure features three quaternary ammonium linkers at the base and three chiral cyclohexanediamine units positioned at the rim. Acting as a supramolecular chiral ligand, the coordination of this cage with copper salts forms an optimal chiral environment that enables an efficient decarboxylative Mannich reaction between β-ketoacids and imines, yielding a broad range of chiral β-amino carbonyl compounds. Mechanistic studies and control experiments reveal that the coordinated Cu center is responsible for the substrate grabbing and preorganization within the cavity and the free NH group contributes to the enhanced enantioselectivity through hydrogen bonds, collaboratively enhancing the overall catalytic efficiency.