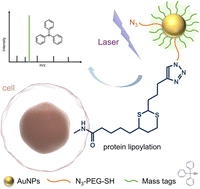
Protein lipoylation is a post-translational modification (PTM) of great significance as the lipoylation sites have essential effects on the enzymatic activities of several protein complexes, which affect the biological metabolic pathways and are further related to some diseases, such as cancer and Alzheimer’s disease. While proteomic identification of lipoylated proteins has been studied, in situ profiling of protein lipoylation with high sensitivity remains challenging. Herein, we developed a strategy for in situ analysis of protein lipoylation by laser desorption/ionization-mass spectrometry (LDI-MS). In this study, a chemoselective butyraldehyde probe (BAP) was used to label the lipoylated proteins, and then linked with laser cleavable probe modified gold nanoparticles (AuNPs) through click chemistry. Triphenylmethyl mercaptan was used as mass tags (MTs) for the tertiary carbocations released under laser (355 nm) irradiation, which reflected the presence of protein lipoylation. Based on this strategy, a relative quantitative analysis of protein lipoylation on different cell lines was performed, and the distribution of lipoylated proteins in tissues was revealed by MS imaging (MSI). This novel approach used chemical modification to achieve signal amplification and overcome the low ionization efficiency and complicated mass spectra of standard protein analysis. This approach exhibits promise for uncovering biological processes and application in the diagnosis of related diseases.