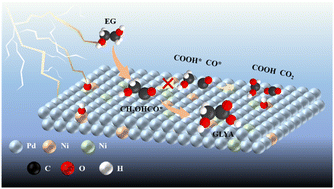
The electrocatalytic oxidation of ethylene glycol (EG) to produce valuable glycolic acid (GLYA) is a promising strategy to tackle EG overcapacity. Despite the good selectivity of Pd for EG oxidation, its performance is constrained by limited mass activity and toxicity of intermediates like CO or CO-analogues. This study reports the alloying of Pd with Ni and Mo metals to enhance the activity and durability of EG oxidation in alkaline media. Notably, the peak current density reached up to 2423 mA mg−1, double that of pristine Pd/C, accompanied by a GLYA Faraday efficiency up to 87.7%. Moreover, PdNiMo/C exhibited a 5-fold slower activity decline compared to Pd/C. In situ experiments and theoretical analysis reveal that Ni and Mo synergistically strengthen the oxygen affinity of the catalyst, facilitating the generation of *OH radicals at lower potentials, thereby accelerating EG oxidation kinetics. Additionally, Ni incorporation prevents C–C bond cleavage and weakens CO adsorption, effectively mitigating catalyst poisoning.