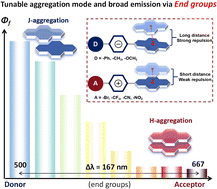
To clarify the impact of the end groups on aggregation behaviour and emission properties, a series of α-cyanostilbene pyrene-based compounds (Py-R and Py-PR) were synthesized. These compounds display aggregation-induced emission characteristics with broad colour-tunable solid-state emission with the colour ranging from cyan to deep red (500–667 nm), achieved by modulating their end groups. The electronic effect of these end groups plays a key role in regulating the emission and the aggregation mode, demonstrating a strong correlation with Hammett constants. Specifically, as end groups transition from donor to acceptor substituents, the aggregates gradually shift from J-aggregation to H-aggregation. This transition is accompanied by a gradual red shift in emission wavelength and a decrease in quantum yield. This regulatory mechanism is realized by adjusting the electrostatic potential on the aromatic ring through the end groups, which in turn affects the centroid distance between adjacent π planes and intermolecular interactions in the aggregated state. Moreover, these AIEgens demonstrate remarkable photochromism through a continuous Z → E transformation and a [2 + 2] cycloaddition reaction. Leveraging these fascinating photophysical properties, selected green, yellow and red AIEgens have been successfully applied for anti-counterfeiting. This work demonstrates precise control over end-group effects on aggregation modes and emission behaviour, elucidates the underlying regulatory mechanism, and underscores its significance for tailoring advanced functional materials.