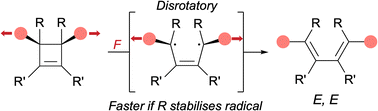
Symmetry-forbidden reactions are notoriously difficult to investigate as they are typically overshadowed by the corresponding symmetry-allowed pathway. Mechanical activation allows access to reaction pathways disfavoured using other methods of activation, such as the symmetry-forbidden disrotatory ring-opening of substituted cis-cyclobutenes. In a recent publication, Bowser, et al. have studied the effects of various substituents on this reaction using atomic force microscopy and computational analysis (B. H. Bowser, C. L. Brown, J. Meisner, T. B. Kouznetsova, T. J. Martínez and S. L. Craig, Chem. Sci., 2025, https://doi.org/10.1039/D5SC00253B). The largest effect is observed with substituents close to the scissile bond having the ability to stabilise the diradical character of the disrotatory ring-opening reaction pathway.