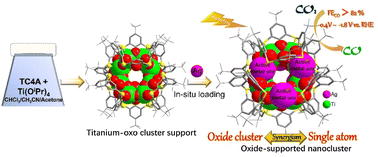
Precise control over the distribution of active metal sites on catalyst surfaces is essential for maximizing catalytic efficiency. Addressing the limitations of traditional cluster catalysts with core-embedded catalytic sites, this work presents a strategy to position catalytic sites on the surfaces of oxide clusters. We utilize a calixarene-stabilized titanium–oxo cluster (Ti12L6) as a scaffold to anchor Ag1+ in situ, forming the unique nanocluster Ti12Ag4.5 with six surface-exposed Ag1+ sites. The in situ transformation from Ti12L6 into Ti12Ag4.5 clusters was traced through mass spectrometry, revealing a solvent-mediated dynamic process of disintegration and reassembly of the Ti12L6 macrocycle. The unique Ti12Ag4.5 cluster, featuring a surface-exposed catalytic site configuration, efficiently catalyzes the electroreduction of CO2 to CO over a broad potential window, achieving CO faradaic efficiencies exceeding 82.0% between −0.4 V and −1.8 V. Its catalytic performance surpasses that of bimetallic Ti2Ag2, which features a more conventional design with Ag1+ sites embedded within the cluster. Theoretical calculations indicate that the synergy between the titanium–oxo support and the single Ag1+ sites lowers the activation energy, facilitating the formation of the *COOH intermediate. This work reveals that engineered interactions between active surface metal and the oxide support could amplify catalytic activity, potentially defining a new paradigm in catalyst design.