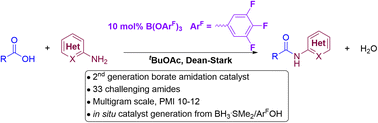
The catalytic activity of different classes of boron catalysts was studied in amidation reactions with 4-phenylbutylamine/benzoic acid, and with 2-aminopyridine/phenylacetic acid. Whilst a simple boronic acid catalyst showed high catalytic activity with the former substrates, it was completely inactive in the latter reaction. In contrast, a borate ester catalyst was able to mediate the amidation of both substrate pairs with moderate activity. By screening a range of borate esters we were able to identify a novel borate catalyst that shows high reactivity with a range of challenging carboxylic acids/amine pairs, enabling catalystic amidation reactions to be achieved effectively with these industrially relevant compounds. The reactions can be performed on multigram scale with high levels of efficiency, and in situ catalyst generation from commercially available reagents renders the process readily accessible for everyday laboratory use. Further experiments showed that the deactivating effect of 2-aminopyridine on boronic acid catalysts was due to its ability to stabilise catalytically inactive boroxines.