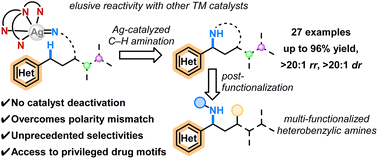
We report a method for the site- and stereoselective intramolecular amination of electron-deficient heterobenzylic C–H bonds via silver-catalyzed nitrene transfer (NT). A silver complex supported by a tripodal piperidine-based ligand afforded excellent reactivity under mild conditions (up to 96% yield), site-selectivity (up to >20 : 1), and diastereoselectivity (up to >20 : 1 dr) for the amination of heterobenzylic C–H bonds that reacted poorly with other metal-based catalysts for NT. Our catalyst proved highly amenable to substrates bearing diverse competing sites for functionalization, including complex molecules derived from pharmaceuticals and natural products. Ligand screening revealed the importance of scaffold rigidity, leading to the discovery of an analogous quinuclidine-based ligand that further improved the site-selectivity against tertiary C–H bonds in a handful of challenging substrates. The cyclic sulfamate products were readily converted into highly functionalized motifs containing nitrogen-based heterocycles and diverse functional groups. Mechanistic studies suggested a radical-based pathway displaying relatively low sensitivity towards the electronic profiles of the heterobenzylic C–H bond, which contributes to the excellent substrate tolerance of this method.