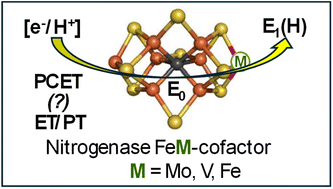
Nitrogenase catalyzes biological nitrogen fixation, the conversion of atmospheric N2 into bioavailable ammonia. The three nitrogenase isozymes—Mo-nitrogenase, V-nitrogenase, and Fe-nitrogenase—utilize catalytic cofactors distinguished by their metal composition (Fe7M, M = Mo, V, or Fe; denoted FeM-co). Their catalytic cycles involve stepwise addition of 8[e−/H+] to FeM-co, generating intermediates designated En, where n is the number of [e−/H+] delivered. The electron-transfer has been extensively characterized, but the proton delivery has not. Here, we investigate [e−/H+] delivery during early-stage conversions, primarily E0 → E1(H), for each of the three nitrogenases, using as reductants γ-ray-generated thermolyzed, mobile electrons at 77 K, and radiation-generated solvent radicals during subsequent annealing to higher temperatures. Our results show E0 → E1(H) conversion differs among the three MFe-proteins. The FeMo-co of MoFe-protein accepts an electron (ET) during 77 K γ-irradiation, but proton transfer (PT) to generate E1(H) is only enabled by conformational or thermodynamic activation upon cryoannealing to ∼200 K(ET/PT). For VFe-protein, E1(H) forms during annealing at-and-above 210 K by electron-transfer to FeV-co from radicals through proton-coupled electron transfer (PCET), which too is enabled by activated proton transfer. FeFe-protein differs in directly exhibiting delivery of protons at 77 K, which together with the mobile electrons react to form E1(H). This could well occur by PCET at 77 K, but does not preclude the possibility of sequential 77 K electron/proton transfer (ET/PT). In addition, 450 nm photolysis reveals the E1(H) state of FeV-co, like that of FeFe-co, contains a hydride bound to a formally oxidized cofactor. The mechanistic differences observed here provide a contribution towards understanding the sources of catalytic differences among the three nitrogenase isozymes.