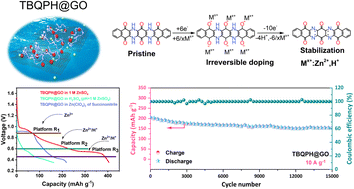
N-type organic cathode materials containing carbonyl and imine groups have emerged as promising candidates for zinc-ion batteries due to their excellent charge storage capability, which arise from the synergic storage of both Zn2+ and H+. However, an increase in active sites also complicates the synthesis, introduces complex multi-electron reactions, and hinders comprehensive understanding of the charge storage mechanism and the evolution of molecular configuration during the electrochemical process. Herein, a 10-electron transfer organic cathode material, featuring imine and quinone groups that are spaced apart, was synthesized in one-step. Its highly conjugated molecular structure promotes electron delocalization, thereby enhancing the stability. The competitive storage mechanism of Zn2+ and H+ was unveiled through multiple quasi in situ spectroscopy techniques and calculations, revealing that Zn2+ are initially coordinated to form O–Zn–N, followed by the co-insertion of H+/Zn2+ during the reduction of the carbonyl groups. Thanks to the Zn2+/H+ co-insertion and coordination stabilization, an ultra-high capacity of 445 mA h g−1 at a current density of 0.2 A g−1 and a retained capacity of 200 mA h g−1 (>80% capacity retention) at 10 A g−1 after 15 000 cycles can be achieved. The molecular structure-related charge storage mechanism revealed in this study can provide useful design considerations for realizing high-capacity, fast-charging and long-duration organic cathodes for various energy storage systems.