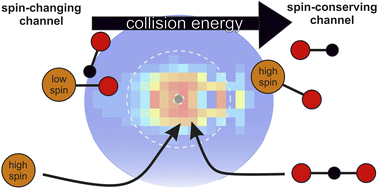
Multi-state reactivity is a well-established concept to explain the surprising reactivity of open-shell systems when a spin-conserving channel is energetically inaccessible. Under such circumstances, the reaction is facilitated by intersystem crossing between different spin manifolds. Advancing the molecular-level understanding of multi-state reactivity requires well-defined model systems, which can be experimentally and theoretically treated at the required level of detail. The oxygen atom transfer from CO2 to a transition metal cation in the gas phase presents such a prototype. Here, we present a joint experimental and theoretical study on the reaction dynamics and kinetics of Zr+ + CO2. Experimental energy and angle-resolved differential cross sections reveal dominant indirect atomistic dynamics in accord with recent studies on similar reactions with tantalum and niobium cations. Interestingly, trajectory simulations on full-dimensional coupled potential energy surfaces of the lowest-lying quartet and doublet electronic states of the Zr+ + CO2 → ZrO+ + CO system from machine-learned first-principles data reveal a competition between an exothermic intersystem crossing pathway to 2ZrO+ and an endothermic spin-conserving channel to 4ZrO+. The experimental product ion velocity distributions are consistent with a switch of the dominant reaction channel when it is energetically allowed. The integral cross sections and thermal rate coefficients at low collision energies reveal three regimes with regard to the energy dependence of the integral cross section. A shift in energy dependence from E−0.5 at the lowest energies at sub-Langevin values to E−1 at intermediate energies, and finally to a positive energy dependence when the spin-conserving channel opens. These behaviors are well-explained by non-adiabatic transition-state theory and are distinctive of a submerged crossing point. The present study highlights the importance of spins revealing a delicate balance of their individual contributions to the interaction potential and resulting reaction dynamics.