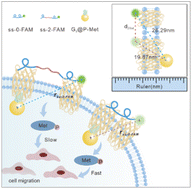
Nanoscale dimerization geometries dictate transmembrane receptor phosphorylation dynamics, yet current methods fail to directly correlate spatial receptor organization with real-time phosphorylation states in situ. Receptor tyrosine kinase (RTK) activation, essential for cellular signaling, is dynamically controlled by dimerization at the nanoscale, but existing tools cannot simultaneously resolve spatial dimer configurations and phosphorylation activity. Here, we developed a transmembrane nanogold surface energy transfer ruler (T-Nanoruler) to enable in situ tracking of RTK activation. This system integrates extracellular DNA aptamers (ss-n-FAM) that program receptor dimerization spacer (0–8.6 nm) with intracellular gold nanoparticle–antibody conjugates (G5@P-Met) targeting phosphorylated residues, creating a molecular ruler for nanoscale distance quantification. Using Met as a model, flow cytometry revealed distance-dependent quenching efficiencies that precisely matched phosphorylation levels validated by western blotting and scratch wound assays. Tight dimerization (0 nm spacer) maximized phosphorylation and cell migration, whereas extended configurations (8.6 nm spacer) suppressed activation. T-Nanoruler overcomes the limitations of FRET (limited detection range) and conventional ex situ methods (poor spatiotemporal resolution), establishing a versatile framework for probing spatial regulation across diverse RTK signaling systems. Our results established the first quantitative paradigm for synchronously mapping phosphorylation dynamics and dimerization geometries with nanometer precision, offering unprecedented insights into allosteric transmembrane signaling mechanisms.