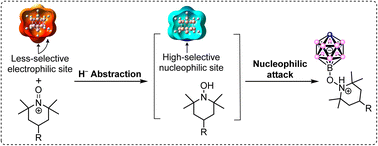
Highly regioselective B(12) substitutions of the monocarborane anion [CB11H12]− has been a challenge. Here, we synthesized a stable B–O–N zwitterionic compound with an impressive yield (isolated yield up to 98%) and excellent regioselectivity at the B(12) position under catalyst-free conditions. The kinetics, substituent effect, and capture experiments are paired with theoretical calculations, showing that the reaction mechanism is oxidation-induced nucleophilic substitution. The hydride anion at the B(12) position is abstracted by an oxoammonium oxidant with lower cleavage energy of 4.2 kcal mol−1 than B(7–11) positions, thereby changing the electronegativity upon the conversion of [CB11H12]− to neutral [CB11H11], in turn giving very high regioselectivity for nucleophilic substitution. This work presents an effective method for synthesizing B(12) oxygen derivatives of the [CB11H12]− anion.
![Graphical abstract: Oxidation-induced nucleophilic substitution at the electron-rich B(12) vertex in [CB11H12]− under catalyst-free conditions](http://pubs.rsc.org/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=D5SC00234F&imageInfo.ImageIdentifier.Year=2025)