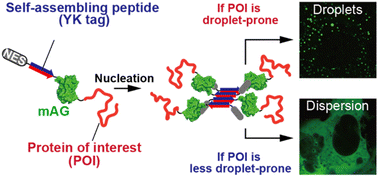
Protein droplet formation is a crucial process involved in transient cellular responses and pathogenic protein aggregations. Conventionally, the droplet-forming capability of target proteins has been evaluated through in vitro reconstitution studies, where purified proteins are dissolved in buffer solutions. However, such droplets are highly sensitive to environmental factors, including temperature, ionic strength, and molecular crowding. Therefore, in situ evaluation within living cells is highly desirable. Additionally, since droplet formation is typically initiated by nucleation involving dynamic protein oligomerization, simply expressing proteins in cells often fails to induce droplet formation, making intracellular evaluation challenging. In this study, we present an intracellular droplet-forming assay based on our peptide tag technique. This system employs short self-assembling YK peptide tags (7–15 residues), genetically fused to target proteins, to artificially induce oligomerization. Using this approach, we discover that the co-chaperone Hsp70/Hsp90 organizing protein possesses droplet-forming capability and identify the essential region required for its droplet formation.