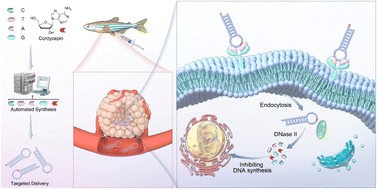
Cordycepin (3′-deoxyadenosine, 3′-dA), derived from the fungus Cordyceps sinensis, has shown significant bioactivity as an inhibitor of enzymes related to 2′-deoxyadenosine (dA). However, its therapeutic efficacy is insufficient for clinical use, which may be addressed through targeted delivery systems. In this study, we designed and synthesized a 3′-dA phosphoramidite to incorporate cordycepin into the well-known cancer-targeting Sgc8c aptamer, where it functions both as a structural modulator and as a bioactive drug element for constructing aptamer–drug conjugates. Its structural similarity to dA makes cordycepin a unique molecular tool for probing the structure–activity relationship of aptamers. Additionally, cordycepin can be seamlessly integrated into aptamers, replacing dA. This led to the generation of a series of cordycepin-modified aptamers, among which Sgc8-23A demonstrated enhanced antitumor activity against HCT116 human colon cancer cells. Compared to free cordycepin, Sgc8-23A exhibited superior bioactivity and stability. In a zebrafish patient-derived xenograft (PDX) model, Sgc8-23A significantly inhibited tumor growth, highlighting its potential as an effective aptamer–drug conjugate for targeted cancer therapy. These findings emphasize the dual functional potential of cordycepin as both a structural element for aptamer optimization and a therapeutic drug component, paving the way for the development of more efficient aptamer-based drug delivery systems.