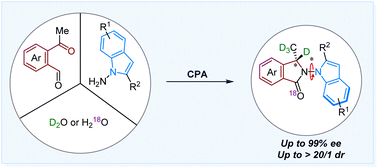
Isotopically chiral molecules have drawn much attention due to their practical applications in drug discovery. However, existing studies in this area are mainly limited to centrally chiral molecules and H/D exchange. Herein, we report a chiral phosphoric acid-catalyzed atroposelective [4+1] annulation of ketoaldehydes and 1H-indol-1-amines. By means of this strategy, a series of D- and 18O-labeled atropisomers featuring both central and axial chiralities are synthesized with high enantioselectivities and diastereoselectivities and good to excellent isotopic incorporation. Experimental and density functional theory studies suggest that the reaction involves a sequential condensation, cyclization and isomerization cascade, in which the second step is the enantio-determining process.
![Graphical abstract: Atroposelective [4+1] annulation for the synthesis of isotopic isoindolinones bearing both central and axial chirality](http://pubs.rsc.org/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=D5SC00594A&imageInfo.ImageIdentifier.Year=2025)