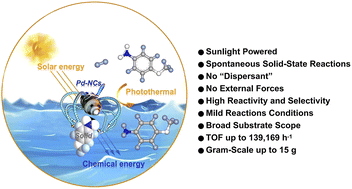
The rate-limiting step in solid-state reactions involves the diffusion of atoms, molecules, or ions through the crystalline phases of the reactant, intermediate, and product. This process is slow, often requiring days or even weeks of continuous or intermittent treatment, while consuming a significant amount of energy. This study describes a light-driven spontaneous solid-state synthesis strategy for the preparation of solid aromatic amines. Under ambient conditions (25 °C, 1 atm H2), natural light irradiation (≥100 W) triggers surface plasmon resonance in 12R-Pd-NCs, inducing directional adsorption of solid nitroarenes and facilitating spontaneous ultrafast electron transfer through non-mechanochemical pathways. The system achieves exceptional efficiency with product yields exceeding 99% and chemical selectivity >99% for aromatic amines. Gram-scale experiments (15 g substrate) reveal remarkable catalytic performance, exhibiting a turnover number (TOF) of 1.39 × 105 h−1 while maintaining full catalytic activity through five consecutive cycles. This methodology transcends conventional thermodynamic limitations by establishing a novel “photon-induced electron tunneling-proton-coupled interface” mechanism in solid-state reactions, opening new avenues for sustainable chemical transformations.