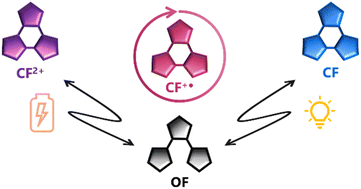
Multi-addressable molecular photoswitches whose isomerisation can be triggered not only by light, but also by other stimuli are appealing for the development of novel smart materials as well as for broadening the areas for their potential application. Diarylethenes (DAEs) are among the most studied switches for this purpose, since tailored functionalisation can make them responsive not only to UV or visible light, but also to other inputs, such as an electrochemical one. In this work, we synthesised five terarylene-based switches and investigated their photochemical and redox properties. Unlike their DAEs analogues, whose cyclisation upon an oxidation-reduction sequence is well-established, our systems undergo a similar oxidative ring-closing of the neutral open form to a doubly charged closed form while the subsequent reduction leads to ring-opening to the neutral open form. Moreover, the neutral closed form can also be re-opened by a catalytic amount of oxidant. With the support of theoretical modelling and cyclic voltammetry simulations, a general mechanism is proposed to rationalise this original bidirectional dual-responsive behaviour.